Igniting The Prebiotic Innovation Engine of Earth's Dead Chemistry
How Ancient Chemical Processes Sparked the Emergence of Complex Life
When exploring the origins of complex life on Earth, we inevitably encounter the famous chicken-or-egg paradox.
In other words, if all cells come from pre-existing cells, how did the first cells originate?
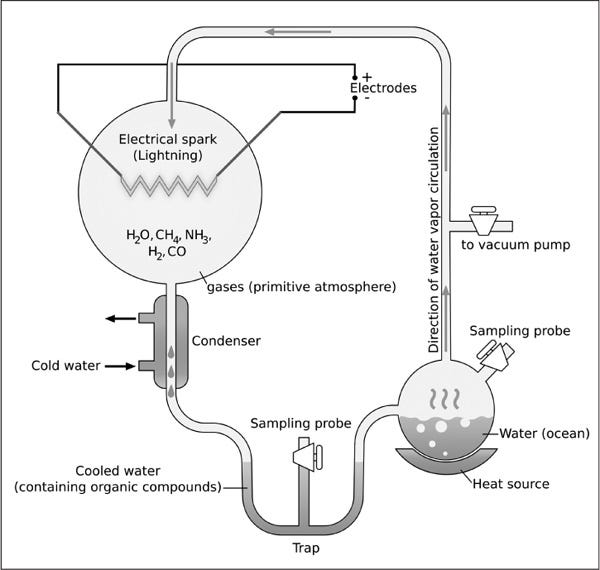
The 1952 Miller–Urey experiment was a groundbreaking experiment that set out to study this very question.
Conducted by Stanley Miller and Harold Urey at the University of Chicago, the experiment used a closed glass system to test whether complex organic molecules—the building blocks of life—could spontaneously form from simpler inorganic compounds under the conditions of the early Earth.
By introducing methane (CH₄), ammonia (NH₃), hydrogen (H₂), and water (H₂O) into the system, they hoped to simulate Earth’s prebiotic atmosphere. After constructing a primitive atmosphere they next shocked the gas with electrical sparks to simulate lightning strikes.
Remarkably, it took just seven days for the electrochemical mixture to spontaneously produce seven of twenty essential amino acids required for complex organic life.
The Random Walk of Carbon Through Recombinatorial Space
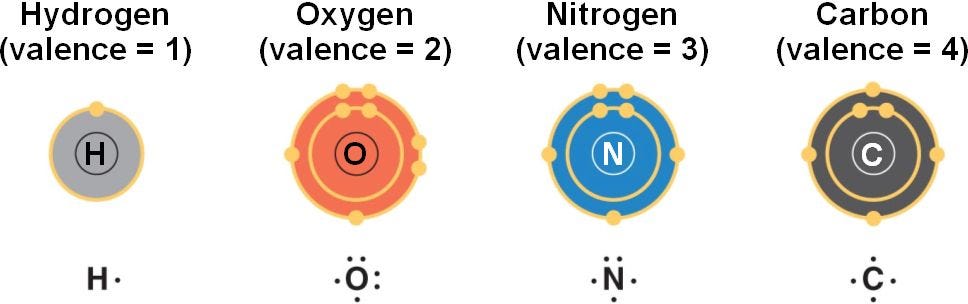
Methane (CH₄), was the only ingredient in the experiment to contain carbon atoms. This was crucial, since carbon's unique structure is necessary for organic life.
Valence electrons are the farthest from the atomic nucleus and the least tightly bound by the powerful forces holding the atom together. Carbon’s high reactivity stems from its four valence electrons, which were ready to interact in early primordial biochemical reactions.
After billions of years of random wandering through the prebiotic chemical space, carbon formed the stable, complex rings and chains that serve as the backbone of all organic life.
Carbon's unique reactivity made it disproportionately important in the structure of organic life. It can bond with 99% of the atoms in organic chemistry, including hydrogen, nitrogen, oxygen, phosphorus, sulfur, and other carbon atoms. As a result, carbon now makes up 20% of the dry weight of humans, despite comprising only 0.2% of the Earth’s crust."
Networks of Matter and The Singular Chemistry of Water
Without the perfect network to traverse, carbon would have failed to form organic life.
Gaseous environments, like the prebiotic atmosphere, lacked the density and stability to encode and propagate spontaneous biochemical structures. Solid surfaces, like Earth's crust, were too rigid for the random interactions needed for complex life to emerge.
However, Earth's saltwater oceans—an ideal 'Goldilocks' network with just the right balance of density, stability, and randomness—enabled the combinatorial exploration, biochemical stabilization, and propagation of carbon’s structures.
Without this perfect water-based environment, carbon would not have been able to form organic life.
Earth's oceans didn't boil away into space because water’s broad liquid temperature range, enabled by its strong hydrogen bonds, kept them intact.
If water weren’t a universal solvent, random elements and molecules would have simply floated on the ocean surface instead of dissolving, preventing carbon from interacting with them. Ocean water is so soluble it can even dissolve gold given enough time.
It’s no surprise that the human body is mostly water—without water’s solvency, biology wouldn’t exist.
Had Earth been burdened with Titan’s methane oceans, it would have remained a realm of dead chemistry forever.
The First Cellular Structures
After billions of years of exploration, carbon found stable biochemical structures with oxygen and phosphate.
Ironically, half of these new molecules—the hydrophobic tail—wanted nothing to do with the surrounding ocean water.
In an effort to escape the water, these molecules spontaneously assembled into tiny molecular bubbles, trapping random samples of the prebiotic soup. But the prebiotic mix was becoming biotic, as carbon-based molecules like amino acids, carbohydrates, and nucleotides began to propagate throughout the oceans.
These bubbles, made of a carbon husk, became the first protocells—the foundation upon which natural selection could build complex organic life.
Encapsulating the biotic soup into cells also compressed the random chemical solutes into a smaller physical space, enhancing their reactivity. The ideal gas law (PV = nRT) tells us that this reduced volume from ocean scale to cell scale increased the pressure and heat, sparking organic life.
After countless random iterations of protocells, simple metabolic pathways spontaneously emerged. The cells developed the ability to replicate, consume other cells for energy, and eject metabolic waste to keep from poisoning themselves to death.
Soon, one prokaryote ingested another, marking the birth of the first predator-prey relationship and the rise of eukaryotes. By consuming the energy of weaker cells, eukaryotes grew larger, more complex, and predatory.
By using a calcium-containing metabolic waste product, eukaryotes built a scaffolding of bones, which were used to hoist themselves out of the oceans for the first time onto dry land.
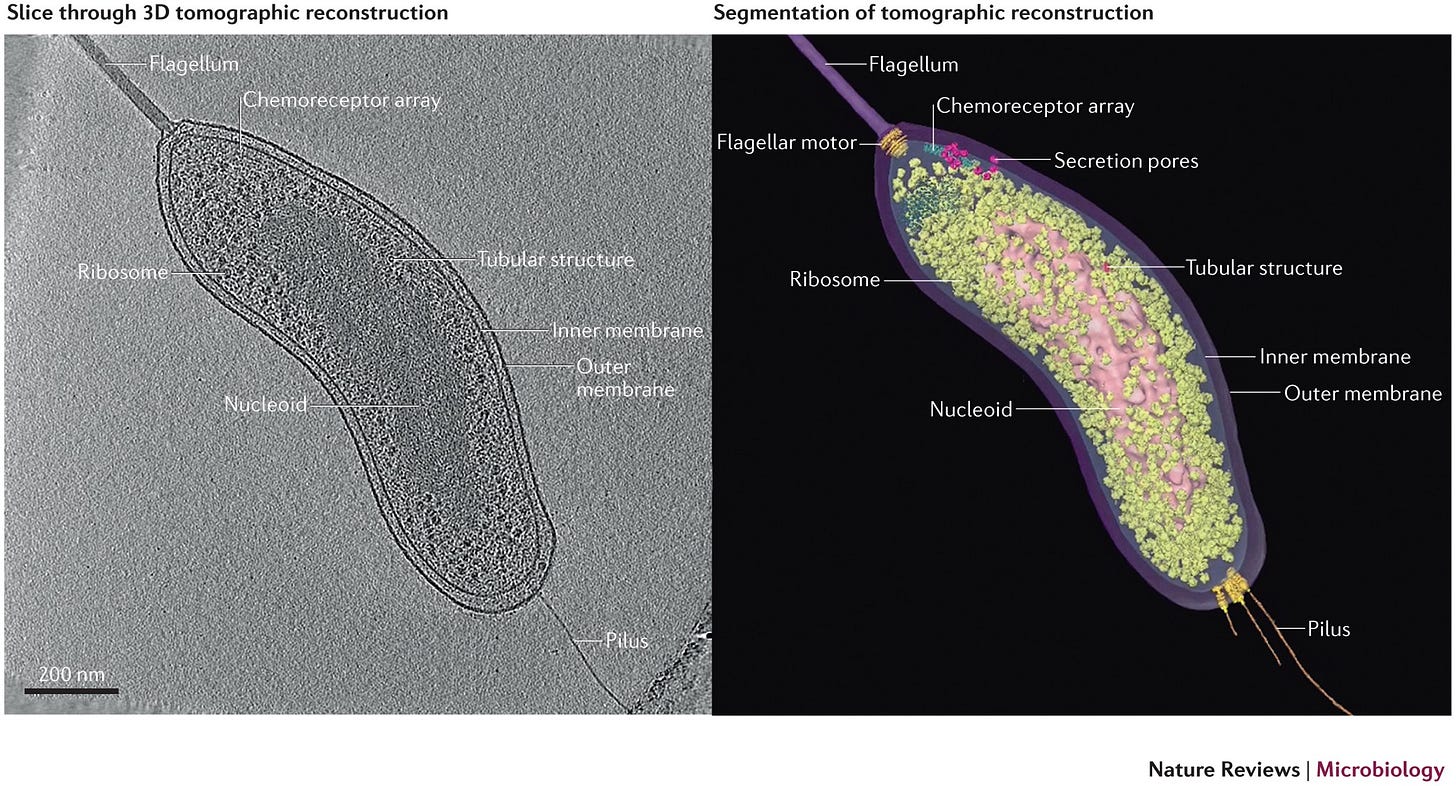
Prokaryotes Under Electron Microscopy
This is the story of a highly reactive atom called carbon, who sifts through a large prebiotic recombinatorial space over billions of years, traversing a goldilocks network of liquid water to self assemble all organic life on Earth.
It’s the story of the first hero to undertake the first hero’s journey.
It’s the shared organic journey we’re all still on, one that continues to unfold with a continuous horizon of uncertainty.
It’s the narrative arc of how atomic physics built dead chemistry which built organic chemistry which built biology which built our human civilization.
“The Creation of Adam” by Michelangelo, 1508-1512, Sistine Chapel, Vatican City